Efficacy and Safety of Fecal Microbiota Transplantation for Parkinson’s Disease: A Systematic Review
Keywords:
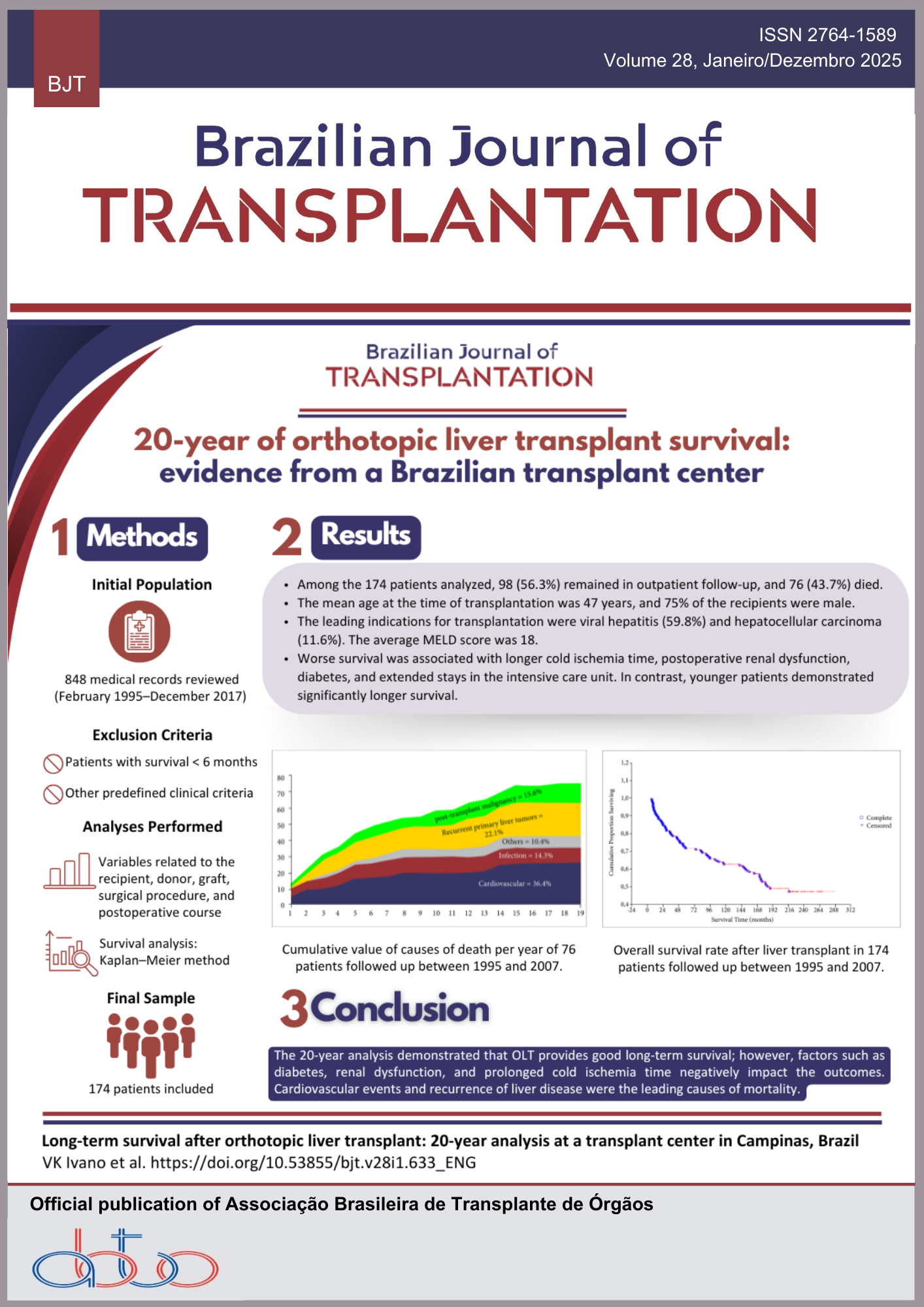
Neurology, Fecal Microbiota Transplantation, Parkinson’s disease, Cognition, Evidence-based medicineAbstract
Background: Fecal microbiota transplantation (FMT) is a procedure that involves transferring fecal material from a healthy donor to a patient to restore intestinal balance. Gut dysbiosis in Parkinson’s disease (PD) worsens motor and gastrointestinal symptoms. Studies suggest that FMT may alleviate these symptoms by improving gut health and reducing neuroinflammation. Methods: This review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Cochrane guidelines. A literature search was performed on Cochrane Library, PubMed, and Scopus databases. Articles were screened for inclusion using the Rayyan® platform, based on predefined eligibility criteria, with any conflicts resolved by consensus. Results: Out of 760 records, four studies met the inclusion criteria. FMT demonstrated variable outcomes, with symptom improvement ranging from 45% to 70% for gastrointestinal disturbances and motor function. Adverse events were minimal, primarily involving mild gastrointestinal discomfort. FMT was effective in restoring gut microbiome balance and reducing neuroinflammation. However, heterogeneity in patient populations, FMT protocols, and study designs complicated the standardization of outcomes. Conclusion: FMT offers a promising therapeutic approach for PD, particularly in improving gastrointestinal and motor symptoms. The variability in patient populations, FMT protocols, and study designs highlights the need for standardized methodologies and more extensive clinical trials. Optimizing FMT administration and exploring its role as an adjunctive treatment alongside conventional therapies could enhance patient outcomes and provide an innovative strategy for managing PD.
Downloads
References
1. Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology, 2013;145(5):946-53. https://doi.org/10.1053/j.gastro.2013.08.058
2. Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol, 2020;10:98. https://doi.org/10.3389/fcimb.2020.00098
3. Cheng Y, Tan G, Zhu Q, Wang C, Ruan G, Ying S, et al. Efficacy of fecal microbiota transplantation in patients with Parkinson’s disease: clinical trial results from a randomized, placebo-controlled design. Gut Microbes, 2023;15(2):2284247. https://doi.org/10.1080/19490976.2023.2284247
4. Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci, 2017;18(7):435-50. https://doi.org/10.1038/nrn.2017.62. Erratum in: Nat Rev Neurosci. 2017;18(8):509. https://doi.org/10.1038/nrn.2017.91
5. Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis, 2013;50:42-8. http://doi.org/10.1016/j.nbd.2012.09.007
6. Bruggeman A, Vandendriessche C, Hamerlinck H, De Looze D, Tate DJ, Vuylsteke M, et al. Safety and efficacy of faecal microbiota transplantation in patients with mild to moderate Parkinson’s disease (GUT-PARFECT): a double-blind, placebocontrolled, randomised, phase 2 trial. EClinicalMedicine, 2024;71:102563. https://doi.org/10.1016/j.eclinm.2024.102563
7. Scheperjans F, Levo R, Bosch B, Lääperi M, Pereira P, Smolander OP, et al. Fecal microbiota transplantation for treatment of parkinson disease: a randomized clinical trial. JAMA Neurol, 2024;81(9):925-38. https://doi.org/10.1001/jamaneurol.2024.2305
8. Cochrane Training. Cochrane handbook for systematic reviews of interventions. AvailabChichester (UK): John Wiley & Sons; 2019.
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 2021;372. https://doi.org/10.1136/bmj.n71
10. Higgins JPT, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 2011;343. https://doi.org/https://doi.org/10.1136/bmj.d5928
11. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ, 2016;355:i4919. https://doi.org/10.1136/bmj.i4919
12. DuPont HL, Suescun J, Jiang ZD, Brown EL, Essigmann HT, Alexander AS, et al. Fecal microbiota transplantation in Parkinson’s disease – A randomized repeat-dose, placebo-controlled clinical pilot study. Front Neurol, 2023;14:1104759. https://doi.org/10.3389/fneur.2023.1104759
13. Jiao F, Zhou L, Wu Z. The microbiota-gut-brain axis: a potential target in the small-molecule compounds and gene therapeutic strategies for Parkinson’s disease. Neurol Sci, 2024;46. https://doi.org/10.1007/s10072-024-07878-x
14. Chui ZSW, Chan LML, Zhang EWH, Liang S, Choi EPH, Lok KYW, et al. Effects of microbiome-based interventions on neurodegenerative diseases: a systematic review and meta-analysis. Sci Rep, 2024;14(1):9558. https://doi.org/10.1038/s41598-024-59250-w
15. Dutta SK, Verma S, Jain V, Surapaneni BK, Vinayek R, Phillips L, et al. Parkinson’s disease: the emerging role of gut dysbiosis, antibiotics, probiotics, and fecal microbiota transplantation. J Neurogastroenterol Motil, 2019;25(3):363-76. https://doi.org/10.5056/jnm19044
16. Fan HX, Sheng S, Zhang F. New hope for Parkinson’s disease treatment: targeting gut microbiota. CNS Neurosci Ther, 2022;28(11):1675-88. https://doi.org/10.1111/cns.13916
17. Mahbub NU, Islam MM, Hong ST, Chung HJ. Dysbiosis of the gut microbiota and its effect on α synuclein and prion protein misfolding: consequences for neurodegeneration. Front Cell Infect Microbiol, 2024;14:1348279. https://doi.org/10.3389/fcimb.2024.1348279
18. Heravi FS, Naseri K, Hu H. Gut microbiota composition in patients with neurodegenerative disorders (Parkinson’s and Alzheimer’s) and healthy controls: a systematic review. Nutrients, 2023;15(20):4365. https://doi.org/10.3390/nu15204365
19. Wang Q, Luo Y, Ray Chaudhuri K, Reynolds R, Tan EK, Pettersson S. The role of gut dysbiosis in Parkinson’s disease: mechanistic insights and therapeutic options. Brain, 2021;144(9):2571-93. https://doi.org/10.1093/brain/awab156
20. Tian H, Ding C, Gong J, Ge X, McFarland LV, Gu L, et al. Treatment of slow transit constipation with fecal microbiota transplantation: a pilot study. J Clin Gastroenterol, 2016;50(10):865-70. https://doi.org/10.1097/MCG.0000000000000472
21. Jiao F, Zhou L, Wu Z. The microbiota-gut-brain axis: a potential target in the small-molecule compounds and gene therapeutic strategies for Parkinson’s disease. Neurol Sci, 2024;46. https://doi.org/10.1007/s10072-024-07878-x
22. Kang Y, Kang X, Zhang H, Liu Q, Yang H, Fan W. Gut microbiota and Parkinson’s disease: implications for faecal microbiota transplantation therapy. ASN Neuro, 2021;13:17590914211016217. https://doi.org/10.1177/17590914211016217
23. Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun, 2018;70:48-60. https://doi.org/10.1016/j.bbi.2018.02.005
24. Kuai XY, Yao XH, Xu LJ, Zhou YQ, Zhang LP, Liu Y, et al. Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb Cell Fact, 2021;20(1):98. https://doi.org/10.1186/s12934-021-01589-0
25. Terra, DAA, Vilela E, Silva R, Leão L, Lima KS, Passos RAF, et al. Structuring a fecal microbiota transplantation center in a university hospital in Brazil. Arq Gastroenterol, 2020;57(4):434-58. https://doi.org/10.1590/S0004-2803.202000000-79
26. Messias LBF, Silva A, Silva CAP da, Silva ER, Silva CRN, Miranda FF. Transplante de microbiota fecal no tratamento da infecção por Clostridium difficile: estado da arte e revisão de literatura. Rev Col Bras Cir, 2018;45(2):e1609. https://doi.org/10.1590/0100-6991e-20181609
27. Xiang S, Ji JL, Li S, Cao XP, Xu W, Tan L, et al. Efficacy and safety of probiotics for the treatment of Alzheimer’s disease, mild cognitive impairment, and Parkinson’s disease: a systematic review and meta analysis. Front Aging Neurosci, 2022;14:730036. https://doi.org/10.3389/fnagi.2022.730036
28. Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr, 2019;38(3):1031-5. https://doi.org/10.1016/j.clnu.2018.05.018
29. Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol, 2015;78(4):522-9. https://doi.org/10.1002/ana.24448
30. Fülling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus neuron. Neuroview, 2019;101(6):998-1002. https://doi.org/10.1016/j.neuron.2019.02.008
31. Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA, 2019;321(2):156-64. https://doi.org/10.1001/jama.2018.20046
32. Holster S, Lindqvist CM, Repsilber D, Salonen A, de Vos WM, König J, et al. The effect of allogenic versus autologous fecal microbiota transfer on symptoms, visceral perception and fecal and mucosal microbiota in irritable Bowel syndrome: a randomized controlled study. Clin Transl Gastroenterol, 2019;10(4):e00034. https://doi.org/10.14309/ctg.0000000000000034
33. Brasil. Ministério da Saúde. Protocolo clínico e diretrizes terapêuticas da doença de Parkinson. Brasília (DF): Ministério da Saúde; 2025.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Isabela Coutinho Faria, Kleuber Arias Meireles Martins, Mariana Menezes Corcinio, Pedro Soares de Oliveira, Gabriella Faria Nogueira, Ana Julia da Silva Oliveira Bittarães, Mariane Otoni Braga

This work is licensed under a Creative Commons Attribution 4.0 International License.