The Use of Microbiological Quality Control Techniques Adopted in a Human Tissue Bank
Keywords:
Tissue Donation, Tissue Transplantation, Biological Quality Control, Innocuous Products, Human Tissue BankAbstract
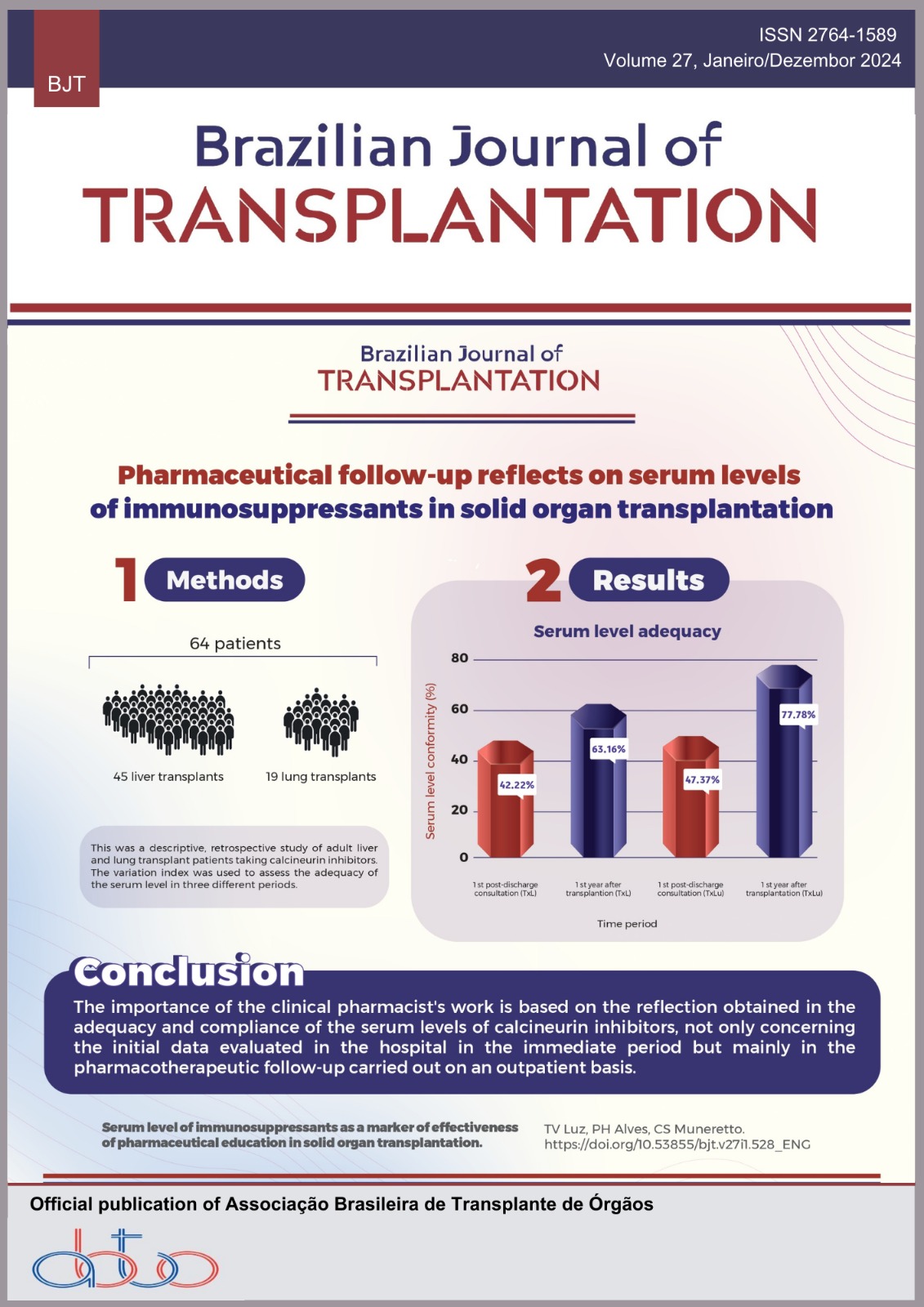
The Brazilian Ministry of Health’s Consolidation Ordinance No. 4 of September 28, 2017, provides the requisites for the evaluation of services for human tissue banks (bancos de tecidos humanos [BTH]) in terms of the microbiological control of environments (ISO 5 classification processing room) and products (culture for aerobic, anaerobic, and fungal pathogens), with a view to guaranteeing harmlessness in tissue transplants. Objectives: To highlight the techniques for microbiological control of distributed tissues adopted by a BTH over 5 years, as well as the results obtained from the application and use of these techniques. Methods: Using standard operating procedures (SOPs), the methodology, and frequency of collections, tests and room cleaning were established in advance. Microbiological cultures were taken from the tissues during all the collections and processing. During tissue processing, fingerprint samples on the processor’s gloves and in the processing room were seeded on blood agar plates. The processing room was cleaned weekly with biguanide and quaternary ammonium. The processing bench was sanitized with sterile 70% alcohol and the microbiological environmental control was carried out every 6 months by a qualified outsourced company. Results: In the period analyzed, the techniques proved effective in 46 (96%) cases, with contamination identified in only two (4%) processed and collected samples. The effectiveness and results were documented. Conclusion: Effective biological quality control methods are legitimately required, but they can be improved, raising the need to develop safe protocols for the quality of services and the safety of transplanted tissues.
Downloads
References
Corsi CA, Shoji M, Scarpelini KC, Bento RL, Becari C, et al. Implementation and certification of ISO 9001: 2015 seal in human tissue bank HCFMRP-USP. Cell Tissue Bank 2020;21:563-71. https://doi.org/10.1007/s10561-020-09852-1
Vialle LRG, Vialle EN, Nianni FN. A technique for the reconstruction of the limbs of osteomuscular tissue donors. Rev Bras Ortop. 2020;55(1):112-4. https://doi.org/10.1055/s-0039-1692696
Corsi CAC, Assunção-Luiz AV, Pitta NC, Cintra AS, Scarpelini KCG, Bento RL, et al. Educational actions to raise student awareness about the donation and transplantation of human organs and tissues. Transplant Proc. 2023;55(6):1329-36. https://doi.org/10.1016/j.transproceed.2023.04.024
Associação Brasileira de Transplante De Órgãos. Registro Brasileiro de Transplantes. Ano XXIX; 2023 [acesso em 14 Jan 2024]. Disponível em: https://site.abto.org.br/wp-content/uploads/2023/12/rbt2023-3trim-naoassociados.pdf
Brasil. Ministério da Saúde. Regulamento nº 9.175, de 18 de outubro de 2017. Regulamenta a Lei nº 9.434, de 4 de fevereiro de 1997, para tratar da disposição de órgãos, tecidos, células e partes do corpo humano para fins de transplante e tratamento. Oficial da República Federativa do Brasil. Brasília (DF): Ministério da Saúde; 1997.
Brasil. Ministério da Saúde. Portaria de Consolidação nº 04, de 28 de setembro de 2017. Consolidação das normas sobre os sistemas e os subsistemas do Sistema Único de Saúde. Oficial da República Federativa do Brasil. Brasília (DF): Ministério da Saúde; 2017.
Brasil. Ministério da Saúde. Resolução da Diretoria Colegiada (RDC) da Agência Nacional de Vigilância Sanitária (ANVISA) nº 707, de 1 de julho de 2022. Dispõe sobre as Boas Práticas em Tecidos humanos para uso terapêutico. Oficial da República Federativa do Brasil. Brasília (DF): Ministério da Saúde; 2022.
Corsi CAC, Assunção-Luiz AV, Cintra AS, Scarpelini KCG, Bento RL, Garcia FL, et al. The significance of the nucleic acid test (NAT) to prevent viral contamination in musculoskeletal tissue transplantation. Rev Bra Ortop. 2023;58(1):23-9. https://doi.org/10.1055/s-0042-1756156
Oliveira MA, Vellarde GC, Sá RAM. Entendendo a pesquisa clínica IV: estudos de caso controle. Femina. 2015:43(4)175-80. 10. Hentz NG. Pharmaceutical bioburden testing. In: Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. New York: Wiley 2009. p. 757-74.
Arendrup MC, Jørgensen KM, Guinea J, Lagrou K, Chryssanthou E, Hayette MP, et al. Multicentre validation of a EUCAST method for the antifungal susceptibility testing of microconidia-forming dermatophytes. J Antimicrob Chemother. 2020;75(7):1807-19. https://doi.org/10.1093/jac/dkaa111
Paolin A, Romualdi C, Romagnoli L, Trojan D. Analysis of potential factors affecting allografts contamination at retrieval. Cell Tissue Bank 2017;18(4):539-45. https://doi.org/10.1007/s10561-017-9667-9
Paolin A, Trojan D, Petit P, Coato P, Rigoli R. Evaluation of allograft contamination and decontamination at the Treviso Tissue Bank Foundation: a retrospective study of 11,129 tissues. PLoS One. 2017;12(3):e0173154. https://doi.org/10.1371/journal.pone.0173154
Coutinho BS, Ribeiro AD, Oliveira SMB, Miranda MKV, Gouvêa-e-Silva LF. Infecções de sítio cirúrgico em cirurgias ortopédicas de um hospital do estado do Pará, Brasil. Av Enferm. 2022;40(3):395-407. https://doi.org/10.15446/av.enferm.v40n3.93397
Novato TF, Wilk MMS, Araújo LT, Abreu EP. Perfil de infecções em artroplastia de quadril: uma revisão integrativa. Health Resid. J. 2021;2(10):91-110. http://dx.doi.org/10.51723/hrj.v2i10.145
Forsell JH, Liesman J. Analysis of potential causes of positive microbiological cultures in tissue donors. Cell Tissue Bank. 2000;1:111-5. https://doi.org/10.1023/A:1010106214542
Corsi CA, Sares CT, Mestriner F, Michelon-Barbosa J, Dugaich VF, Martins TV, et al. Isolation and primary culture of human abdominal aorta smooth muscle cells from brain-dead donors: an experimental model for vascular diseases. Cell Tissue Bank. 2023;25(5):1-8. https://doi.org/10.1007/s10561-023-10091-3
Lannau B, Van Geyt C, Van Maele GE, Beele H. Analysis of potential factors affecting microbiological cultures in tissue donors during procurement. Cell Tissue Bank. 2015;16:65-71. https://doi.org/10.1007/s10561-014-9439-8
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Carlos Alexandre Curylofo Corsi, Katia Carmen Gabriel Scarpelini, Rodolfo Leandro Bento, Alan Vinicius Assunção-Luiz, Flávio Luís Garcia , Luís Gustavo Gazoni Martins

This work is licensed under a Creative Commons Attribution 4.0 International License.