Serum Level of Immunosuppressants as a Marker of Effectiveness of Pharmaceutical Education in Solid Organs Transplantation
Keywords:
Immunosuppression Therapy, Pharmaceutical Attention, Liver Transplant, Lung Transplant, Adherence to MedicationAbstract
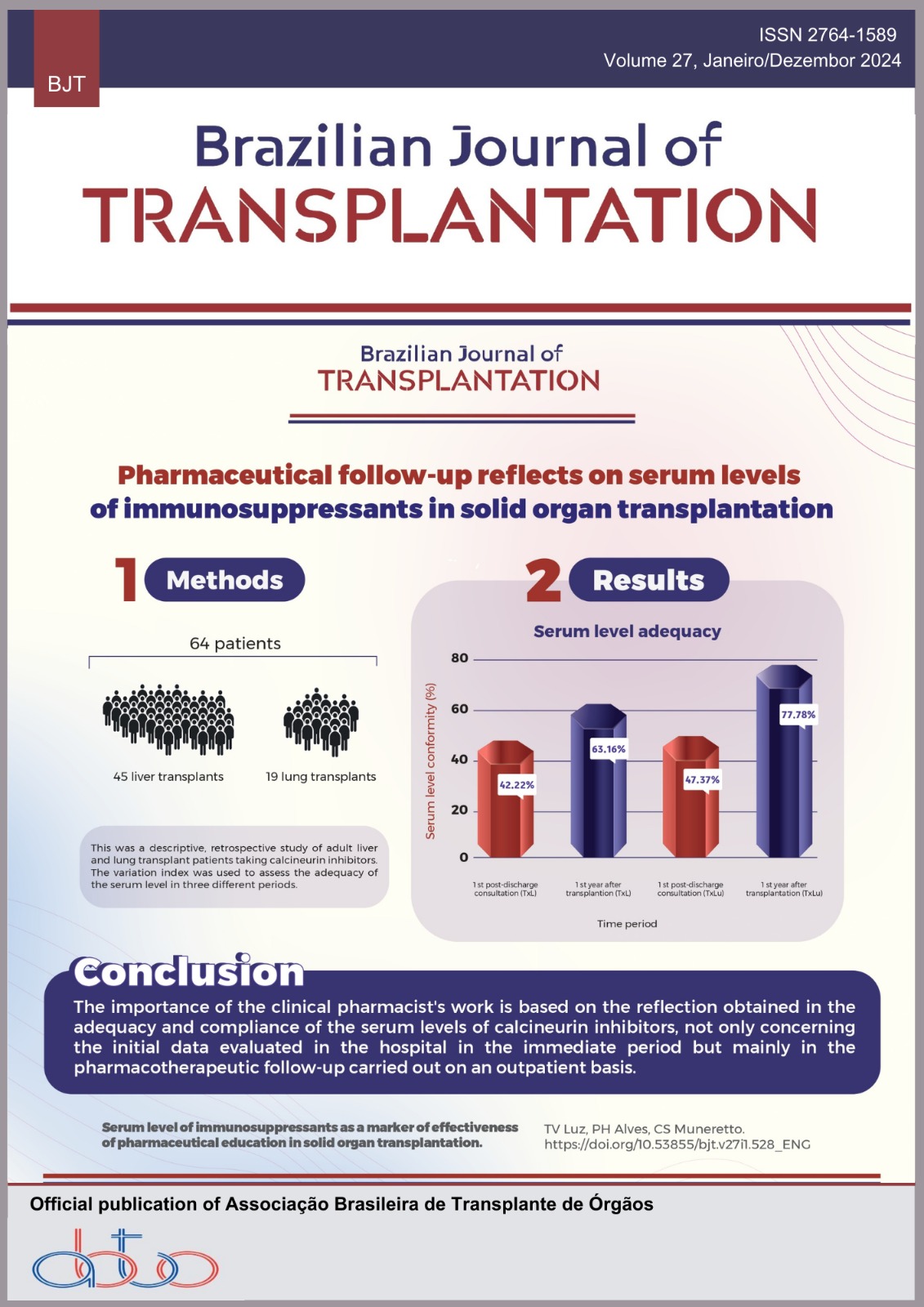
Solid organ transplantation promotes an increase in the quality of life of those with diseases that compromise the functioning of a specific organ, maximizing graft survival and reducing the need for retransplantation is essential. Post-transplant medication adherence is one of the factors that influence positive clinical outcomes, with this, the importance of pharmacotherapeutic follow-up at the hospital and outpatient level with the clinical pharmacist is addressed. Objectives: To verify the impact of pharmacotherapeutic guidance and follow-up on the compliance of serum levels of calcineurin inhibitors in lung and liver transplant patients. Methods: Descriptive and retrospective study with adult liver and lung transplant patients using calcineurin inhibitors, aged over 18 years, between 2018 and 2020. The variation index was calculated through the mean and standard deviation of serum levels collected during the index hospitalization, being used to assess the adequacy of serum levels in three different periods. Results: A total of 84 patients were transplanted, including 64 patients (45 liver transplants and 19 lung transplants). In a subgroup analysis, 42.22% of the liver patients and 47.37% of the lung transplant recipients did not have the same serum level in the first post-discharge consultation. After 1 year of transplantation and differentiated outpatient follow-ups, the target serum levels were reached in 63.16% and 77.78% of the studied populations, respectively. Conclusion: The importance of clinical action was demonstrated from the reflection obtained on the adequacy and conformity of serum levels of calcineurin inhibitors, not only at the hospital level but mainly on the pharmacotherapeutic follow-up carried out on an outpatient basis, in which we observed a more expressive value.
Downloads
References
Gambato M., Frigo AC., Castro KIR., Senzolo M., Nadal E., D’Amico F. et al. Who fares worse after liver transplantation? Impact of donor and recipient variables on outcome: data from a prospective study. Transplantation, v.95, n.12, p.1528-1534, 2013. https://doi.org/10.1097/TP.0b013e318292827f
Nankivell BJ, Kuypers DRJ. Diagnosis and prevention of chronic kidney allograft loss. The Lancet, v.378, n.9800, p.1428-1437, 2011. https://doi.org/10.1016/S0140-6736(11)60699-5
Soares LSDS., Brito ESD., Magedanz L., França FA., Araújo WND., Galato D. Transplantes de órgãos sólidos no Brasil: estudo descritivo sobre desigualdades na distribuição e acesso no território brasileiro, 2001-2017. Epidemiologia e Serviços de Saúde, v.29, 2020. https://doi.org/10.5123/S1679-49742020000100014
Black CK., Termanini KM., Aguirre O., Hawksworth JS., Sosin M. Solid organ transplantation in the 21st century. Annals of Translational Medicine, v.6, n.20, 2018.https://doi.org/10.21037/atm.2018.09.68
Garcia CD., Pereira JD., Garcia VD. Doação e transplante de órgãos e tecidos. 1.ed. São Paulo: Segmento Farma, 2015.
Gómez EJ., Jungmann S., Lima AS. Resource allocations and disparities in the Brazilian health care system: insights from organ transplantation services. BMC Health Services Research, v.18, n.1, p.1-7, 2018. https://doi.org/10.1186/s12913-018-2851-1
Associação Brasileira de Transplante de Órgãos. Dados numéricos da doação de órgãos e transplantes realizados por estado e instituição no período de Janeiro/Junho de 2022. Registro Brasileiro de Transplantes, n.2, ano XXVIII. https://doi.org/10.53855/bjt.v26iSuplementar.552
Vilarinho S., Lifton RP. Liver transplantation: from inception to clinical practice.Cell, v.150, n.6, p.1096-1099, 2012. https://doi.org/10.1016/j.cell.2012.08.030
Charlton M., Levitsky J., Aqel B., O’Grady J., Hemibach J., Rinella M. et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation, v.102, n.5, p.727- 743, 2018. https://doi.org/10.1097/TP.0000000000002147
Rayar M., Tron C., Jézéquel C., Beaurepaire J. M., Petitcollin A., Houssel-Debry P. et al. High intrapatient variability of tacrolimus exposure in the early period after liver transplantation is associated with poorer outcomes. Transplantation, v.102,n.3, p.108-114, 2018. https://doi.org/10.1097/TP.0000000000002052
Lemaitre F., Blanchet B., Latournerie M., Antignac M., Houssel-Debry P., Verdier M. C. et al. Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: inside the white blood cells. Clinical Biochemistry, v.48, n.6, p.406-411, 2015. https://doi.org/10.1016/j.clinbiochem.2014.12.018
Schumacher L., Leino AD., Park JM. Tacrolimus intrapatient variability in solid organ transplantation: a multiorgan perspective. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, v.41, n.1, p.103-118, 2021. https://doi.org/10.1002/phar.2480
Dobbels F., De Bleser L., Berben L., Kristanto P., Dupont L., Nevens F. et al. Efficacy of a medication adherence enhancing intervention in transplantation: The MAESTRO-Tx trial. The Journal of Heart and Lung Transplantation, v.36, n.5, p.499-508, 2017. https://doi.org/10.1016/j.healun.2017.01.007
Belaiche S., Décaudin B., Dharancy S., Noel C., Odou P., Hazzan M. Factors relevant to medication non-adherence in kidney transplant: a systematic review. International Journal of Clinical Pharmacy, v.39, n.3, p.582-593, 2017. https://doi.org/10.1007/s11096-017-0436-4
Neuberger JM., Bechstein WO., Kuypers DR., Burra, P., Citterio, F., De Geest, S. et al. Practical recommendations for longterm management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the consensus on managing modifiable risk in transplantation (COMMIT) group. Transplantation, v.101, n.4S, p.S1-S56, 2017. https://doi.org/10.1097/TP.0000000000001651
Fu R., Tajima S., Suetsugu K., Watanabe H., Egashira N., Masuda S. Biomarkers for individualized dosage adjustments in immunosuppressive therapy using calcineurin inhibitors after organ transplantation. Acta Pharmacologica Sinica, v.40, n.2, p.151-159, 2019. https://doi.org/10.1038/s41401-018-0070-2
Scheel JF., Schieber K., Reber S., Stoessel L., Waldmann E., Jank S. et al. Psychosocial variables associated with immunosuppressive medication non- adherence after renal transplantation. Frontiers in psychiatry, v.9, p.23, 2018. https://doi.org/10.3389/fpsyt.2018.00023
Maciel NB., Schwambach KH., Blatt CR. Liver transplantation: tacrolimus blood levels variation and survival, rejection and death outcomes. Arquivos de Gastroenterologia, v.58, p.370-376, 2021. https://doi.org/10.1590/s0004-2803.202100000-62
Kung M., Koschwanez, HE., Painter L., Honeyman V., Broadbent E. Immunosuppressant nonadherence in heart, liver, and lung transplant patients: associations with medication beliefs and illness perceptions. Transplantation, v.93, n.9, p.958-963, 2012. https://doi.org/10.1097/TP.0b013e31824b822d
de Souza e Silva AC., Cardoso Martins BC., Silveira Adriano L.; de França Fonteles MM., Veras Reis PH., Figueiredo Chaves E. Complexidade da farmacoterapia pós-transplante renal: influência na adesão ao tratamento. Revista Eletrônica de Farmácia, v.14, n.3, 2017. https://doi.org/10.5216/ref.v14i3.44894
Asavakarn S., Sirivatanauksorn Y., Promraj R., Ruenrom A., Limsrichamrern S., Kositamongkol P. et al. Systematic pharmaceutical educational approach to enhance drug adherence in liver transplant recipients. Transplantation proceedings, v.48, n.4, p.1202-1207, 2016. https://doi.org/10.1016/j.transproceed.2015.12.100
Hu L., Lingler JH., Sereika M., Burke LE., Malchano DK., Dabbs, AD. et al. Nonadherence to the medical regimen after lung transplantation: a systematic review. Heart & Lung, v.46, n.3, p.178-186, 2017. https://doi.org/10.1016/j.hrtlng.2017.01.006
Schuh MJ., Massoglia, G. Pharmacist impact on tacrolimus serum concentrations in liver transplant patients. The Consultant Pharmacist, v.33, n.5, p.268-272, 2018. https://doi.org/10.4140/TCP.n.2018.268.
Duwez M., Chanoine S., Lepelley M., Vo TH., Pluchart H., Mazet R. et al. Clinical evaluation of pharmacists interventions on multidisciplinary lung transplant outpatients management: results of a 7-year observational study. BMJ open, v.10, n.11, p. e041563, 2020. https://doi.org/10.1136/bmjopen-2020-041563
Ahmadi ZH., Hamidiab H., Eskandaric R., Bhiab M., Haghgooc R., Salamzadehb, J. et al. The potential role of clinical pharmacist in the practice of heart transplantation. International Pharmacy Acta, v.5, n.1, p.e6:1-7, 2022. https://doi.org/10.22037/ipa.v5i1.37635
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Thayse Ventura Luz, Paola Hoff Alves, Camila Silva Muneretto

This work is licensed under a Creative Commons Attribution 4.0 International License.