Agonistas do Receptor de Peptídeo Semelhante ao Glucagon-1 em Transplantados Renais - Estudo Retrospectivo de um Centro Hospitalar
Palavras-chave:
Agonistas do Receptor de Peptídeo Semelhante ao Glucagon-1, Transplante Renal, Diabetes MellitusResumo
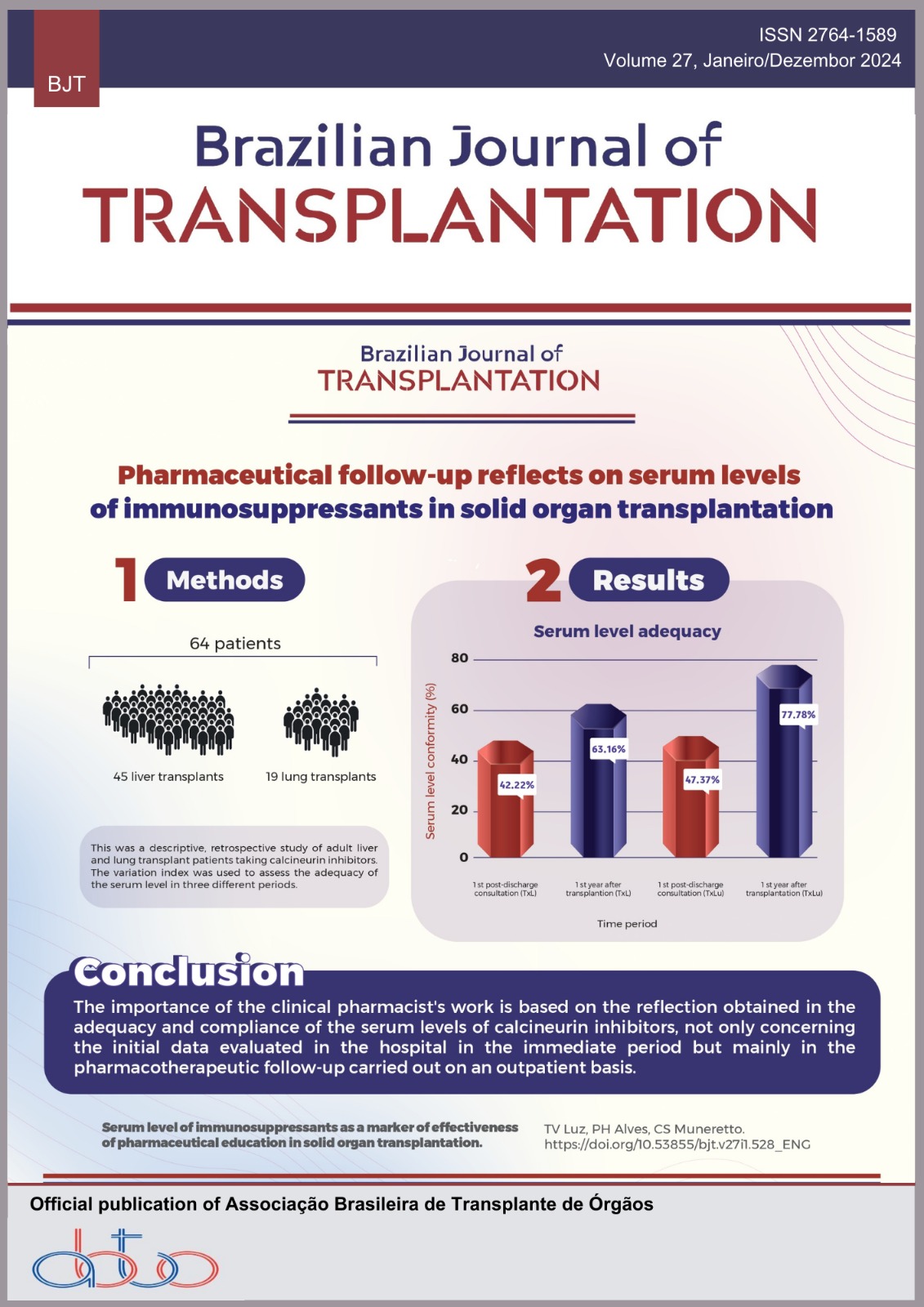
Objetivos: A incidência de diabetes pós-transplante e o aumento do risco cardiovascular entre os receptores de transplante estão em ascensão. Os agonistas do receptor de peptídeo semelhante ao glucagon têm o potencial de mitigar os efeitos dos medicamentos imunossupressores, abordando tanto a hiperglicemia quanto o aumento de peso, o que os torna atrativos para uso nesta população, dadas as suas vantagens cardiovasculares e renoprotetoras. No entanto, a evidência atual é insuficiente sobre a sua eficácia em receptores de transplante renal diabéticos (RTRD). Métodos: O objetivo deste estudo retrospectivo foi avaliar a eficácia e segurança dos agonistas do peptídeo semelhante ao glucagon-1 em RTRD. O foco principal foi avaliar o seu impacto em vários parâmetros, tais como níveis de hemoglobina A1c, índice de massa corporal (IMC), perfil lipídico, níveis de hemoglobina, função do enxerto renal (taxa de filtração glomerular estimada [TFGe]) e relação proteína-creatinina urinária. Resultados: Durante um período de observação mediano de 18 meses, esta investigação incluiu 64 pacientes transplantados renais. A TFGe mediana no início foi de 61,9 mL/min/1,73 m2 e permaneceu estável durante o acompanhamento. A mediana da HbA1c diminuiu de 7,5 para 7% (IC95%; p < 0,002). Também foi observada uma melhoria significativa no IMC e no perfil lipídico. Não foram observadas mudanças significativas nos níveis medianos de creatinina e relação proteína:creatinina urinária. Nenhum efeito colateral justificou a descontinuação do medicamento. Conclusão: Este estudo mostra que o uso de agonistas do peptídeo semelhante ao glucagon é viável e bem tolerado em RTRD, sem efeitos colaterais significativos observados. Estudos subsequentes são necessários para explorar se esta terapêutica pode melhorar efetivamente a sobrevida do aloenxerto nesses pacientes.
Downloads
Referências
Tsai SF, Chen CH. Management of diabetes mellitus in normal renal function, renal dysfunction and renal transplant recipients, focusing on glucagon-like peptide-1 agonist: a review based upon current evidence. Int J Mol Sci 2019;20(13):3152. https://doi.org/10.3390/ijms20133152
Kukla A, Hill J, Merzkani M, Bentall A, Lorenz EC, Park WD, et al. The use of GLP1R agonists for the treatment of type 2 diabetes in kidney transplant recipients. Transplant Direct 2020;6(2):e524. https://doi.org/10.1097/TXD.0000000000000971
Hart A, Smith JM, Skeans MA, Gustafspm SK, Wilk AR, Castro S, et al. OPTN/SRTR 2017 annual data report: kidney. Am J Transplant. 2019;19 Suppl 2:19-123. https://doi.org/10.1111/ajt.15274
Liou JH, Liu YM, Chen CH. Management of diabetes mellitus with glucagonlike peptide-1 agonist liraglutide in renal transplant recipients: a retrospective study. Transplant Proc 2018;50(8):2502-5. https://doi.org/10.1016/j.transproceed.2018.03.087
Andersen A, Lund A, Knop FK, Vilsboll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol 2018;14:390-403. https://doi.org/10.1038/s41574-018-0016-2
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res Clin Pract 2019;157:107843. https://doi.org/10.1016/j.diabres.2019.107843
Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H; International Expert Panel. New-onset diabetes after transplantation: 2003 International Consensus Guidelines. Transplantation 2003;75(10):SS3-24. https://doi.org/10.1097/01.TP.0000069952.49242.3E
Aziz F. New onset diabetes mellitus after transplant: the challenge continues. Kidney360 2021;2(8):1212-4. https://doi.org/10.34067/KID.0004042021
Pham PT, Pham PM, Pham SV, Pham PA, Pham PC. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes 2011;4:175-86. https://doi.org/10.2147/DMSO.S19027
Chakkera HA, Weil EJ, Swanson CM, Dueck AC, Heilman RL, Reddy KS, et al. Pretransplant risk score for new-onset diabetes after kidney transplantation. Diabetes Care 2011;34(10):2141-5. https://doi.org/10.2337/dc11-0752
Boer GA, Holst JJ. Incretin hormones and type 2 diabetes – Mechanistic insights and therapeutic approaches. Biology (Basel) 2020;9(12):473. https://doi.org/10.3390/biology9120473
Pederson RA, Dryburgh JR, Brown JC. The effect of somatostatin on release and insulinotropic action of gastric inhibitory polypeptide. Can J Physiol Pharmacol 1975;53:1200-5. https://doi.org/10.1139/y75-168
Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000;85:3575-81. https://doi.org/10.1210/jcem.85.10.6855
Boer GA, Hartmann B, Holst JJ. Pharmacokinetics of exogenous GIP(1-42) in C57Bl/6 mice; extremely rapid degradation but marked variation between available assays. Peptides 2020:170457. https://doi.org/10.1016/j.peptides.2020.170457
Nauck MA, Kleine N, Ørskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741-4. https://doi.org/10.1007/BF00401145
Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824-30. https://doi.org/10.1016/S0140-6736(02)07952-7
Holst JJ, Ørskov C, Vagn Nielsen O, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 1987;211:169-74. https://doi.org/10.1016/0014-5793(87)81430-8
Montada-Atin T, Prasad GVR. Recent advances in new-onset diabetes mellitus after kidney transplantation. World J Diabetes 2021;12(5):541-5. https://doi.org/10.4239/wjd.v12.i5.541
Mann JFE, Fonseca V, Mosenzon O, Raz I, Goldman B, Idorn T, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation;138(25):2908-18. https://doi.org/10.1161/CIRCULATIONAHA.118.036418
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019, 394: 121-30. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31149-3/abstract
Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, et al. Dulaglutide vs insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6:605-17. https://doi.org/10.1016/S2213-8587(18)30104-9
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834-44. https://doi.org/10.1056/NEJMoa1607141
Pasternak B, Wintzell V, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, et al. Use of glucagon-like peptide 1 receptor agonists and risk of serious renal events: Scandinavian Cohort Study. Diabetes Care 2020;43(6):1326-35. https://doi.org/10.2337/dc19-2088
Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C, et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol (Lausanne) 2021;23(12):721135. https://doi.org/10.3389/fendo.2021.721135
Clemens KK, Ernst J, Khan T, Reichert S, Khan Q; OK TRANSPLANT Investigators. Glucagon-like peptide 1 receptor agonists in end-staged kidney disease and kidney transplantation: a narrative review. Nutr Metab Cardiovasc Dis 2023;33(6):1111-20. https://doi.org/10.1016/j.numecd.2023.03.023
Thangavelu T, Lyden E, Shivaswamy V. A retrospective study of glucagon-like peptide 1 receptor agonists for the management of diabetes after transplantation. Diabetes Ther 2020;11:987-94. https://doi.org/10.1007/s13300-020-00786-1
Pinelli NR, Patel A, Salinitri FD. Coadministration of liraglutide with tacrolimus in kidney transplant recipients: a case series. Diabetes Care. 2013;36:e171-2. https://doi.org/10.2337/dc13-1066
Singh P, Pesavento TE, Washburn K, Walsh D, Meng S. Largest single-centre experience of dulaglutide for management of diabetes mellitus in solid organ transplant recipients. Diabetes Obes Metab 2018;21(4):1061-5. https://doi.org/10.1111/dom.13619
Halden TAS, Egeland EJ, Asberg A, Hartmann A, Midvedt K, Khiabani HZ, et al. GLP-1 restores altered insulin and glucagon secretion in posttransplantation diabetes. Diabetes Care 2016;39(4):617-24. https://doi.org/10.2337/dc15-2383
Mahmoud T, Yagan J, Hasan A, Gheith OA, Mostafa M, Rida S, et al. Sodium-glucose co-transporter 2 inhibitors & glucagonlike peptide-1 receptor agonists, efficacy & safety in diabetic kidney transplant recipients. Clin Transplant 2023;37(12):e15144. https://doi.org/10.1111/ctr.15144
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2024 Joana Freitas, José Silvano, Catarina Ribeiro, Jorge Malheiro, Sofia Pedroso, Manuela Almeida, Isabel Fonseca, La Salete Martins

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.