Inibidores do Cotransportador Sódio-Glicose 2 em Receptores de Transplante Renal Diabéticos: Impacto no Controle Glicêmico, Função do Enxerto e Proteinúria
Palavras-chave:
Proteínas Transportadoras de Sódio-Glicose, Diabetes Mellitus, Transplante Renal, Agentes Imunossupressores, Sobrevivência do EnxertoResumo
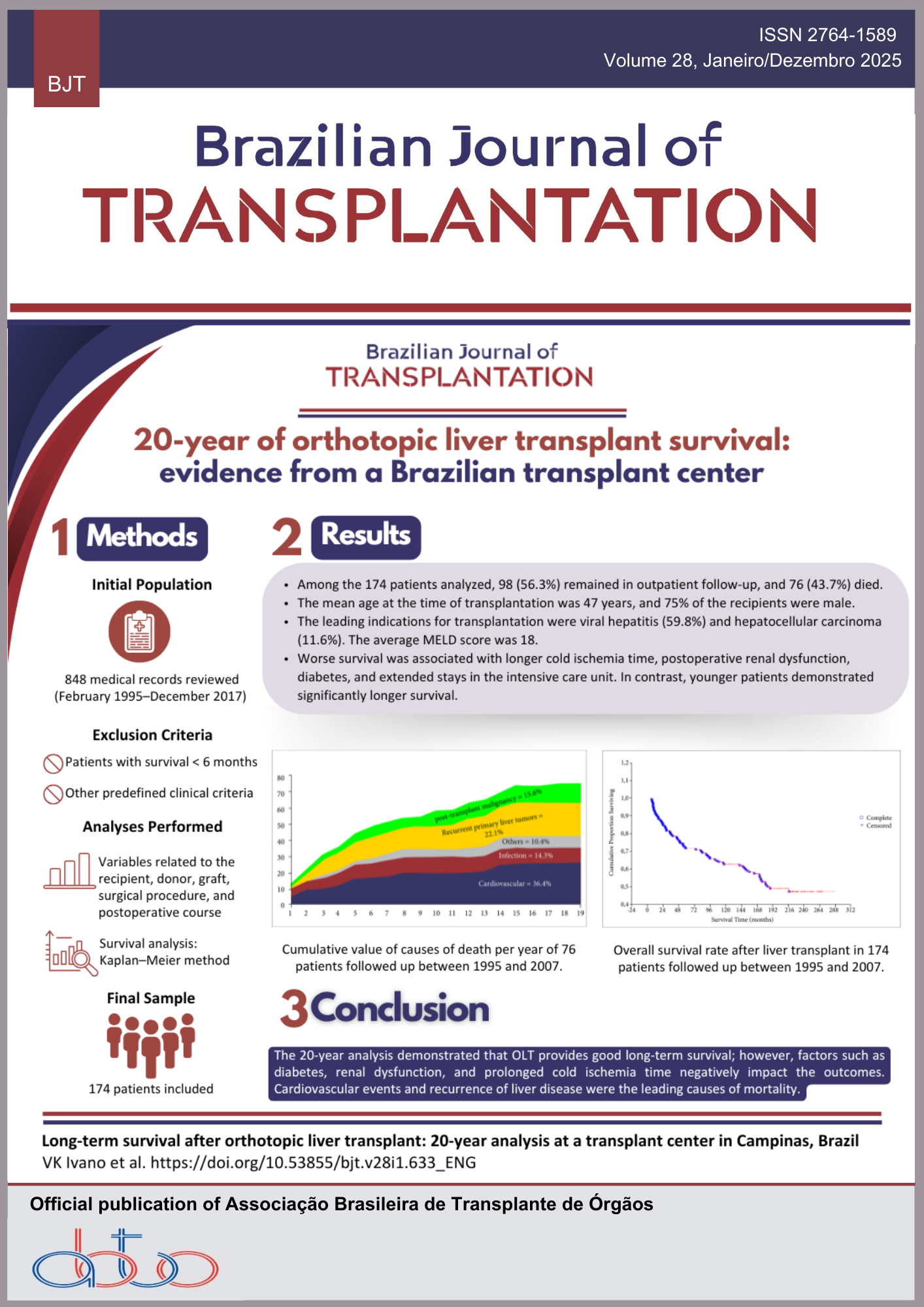
Introdução: Os inibidores do cotransportador sódio-glicose 2 [sodium-glucose cotransporter 2 inhibitors (SGLT2i)] melhoram a albuminúria, a taxa de progressão da doença renal crônica e a morte cardiovascular em pacientes não transplantados com diabetes mellitus (DM) e proteinúria. No entanto, os receptores de transplante renal (RTRs) foram excluídos dos ensaios mais abrangentes. Este estudo teve como objetivo avaliar a função do enxerto, a proteinúria e o controle glicêmico em RTRs com DM pré- e pós-transplante tratados com SGLT2i. Métodos: Este é um estudo unicêntrico, retrospectivo e observacional, incluindo receptores de transplante com mais de 18 anos de idade no momento do transplante, diagnosticados com DM tipo 2 pré-transplante ou DM pós-transplante, tratados com SGLT2i após o transplante de junho de 2020 a junho de 2024. Resultados: Dos 1.883 RTRs acompanhados no centro de junho de 2020 a junho de 2024, 31 receberam SGLT2i, sendo 14 (45,2%) com DM pré-transplante e 17 (54,8%) com DM pós-transplante. Catorze (45,2%) completaram o acompanhamento de 24 meses, incluindo oito (57,1%) no grupo DM pré-transplante e seis (42,8%) no grupo DM pós-transplante. No grupo DM pré-transplante, a glicemia de jejum (GJ) [134 (92-295) mg/dL vs. 109 (73-207), p = 0,24], a taxa de filtração glomerular estimada (TFGe) [59,3 ± 19,2 vs. 68,1 ± 23,4, p = 0,35] e a relação proteína-creatinina na urina (RPCU) [0,3 (0,0-1,9) vs. 0,3 (0,1-0,7), p = 0,80] permaneceram estáveis. No grupo DM pós-transplante, também não houve diferença nos aspectos analisados, seja na GJ [113 (95-225) mg/dL vs. 108 (92-149), p = 0,38], TFGe [73,1 ± 26,8 vs. 69,1 ± 28,9, p = 0,76] ou RPCU [0,2 (0,1-2,5) vs. 0,2 (0,1-0,4), p = 0,21]. Conclusões: Estes resultados sugerem que o tratamento tem efeito benéfico na preservação da função do enxerto ao longo de um período de acompanhamento de 2 anos. O tratamento foi bem tolerado, com baixa incidência de infecção do trato urinário ou disfunção do enxerto.
Downloads
Referências
1. Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis, 2018; 25(2): 121-32. https://doi.org/10.1053/j.ackd.2017.10.011
2. Rossi MR, Mazzali M, de Sousa MV. Oral glucose tolerance test as a risk marker for developing post transplant diabetes mellitus. Transplant Proc, 2024; 56(5): 1061-5. https://doi.org/10.1016/j.transproceed.2024.04.021
3. Chakkera HA, Weil EJ, Pham PT, Pomeroy J, Knowler WC. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care, 2013; 36(5): 1406-12. https://doi.org/10.2337/dc12-2067
4. Paek JH, Kang SS, Park WY, Jin K, Park SB, Han S, et al. Incidence of post-transplantation diabetes mellitus within 1 year after kidney transplantation and related factors in Korean cohort study. Transplant Proc, 2019; 51(8): 2714-7. https://doi.org/10.1016/j.transproceed.2019.02.054
5. Rossi MR, Mazzali M, de Sousa MV. Post-transplant diabetes mellitus: risk factors and outcomes in a 5 year follow-up. Front Clin Diabetes Healthc, 2024; 5. https://doi.org/10.3389/fcdhc.2024.1336896
6. Birdwell KA, Park M. Post-transplant cardiovascular disease. Clin J Am Soc Nephrol, 2021; 16(12): 1878-89. https://doi.org/10.2215/CJN.00520121
7. Rodríguez-Rodríguez AE, Porrini E, Hornum M, Donate-Correa J, Morales-Febles R, Khemlani Ramchand S, et al. Posttransplant diabetes mellitus and prediabetes in renal transplant recipients: an update. Nephron, 2021; 145(4): 317-29. https://doi.org/10.1159/000514288
8. Lin H, Yan J, Yuan L, Qi B, Zhang Z, Zhang W, et al. Impact of diabetes mellitus developing after kidney transplantation on patient mortality and graft survival: a meta-analysis of adjusted data. Diabetol Metab Syndr, 2021; 13(1): 126. https://doi.org/10.1186/s13098-021-00742-4
9. Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int, 2018; 94(1): 26-39. https://doi.org/10.1016/j.kint.2017.12.027
10. Montada-Atin T, PrasadGVR. Recent advances in new-onset diabetes mellitus after kidney transplantation. World J Diabetes, 2021; 12(5): 541-55. https://doi.org/10.4239/wjd.v12.i5.541
11. Pollock C, Stefánsson B, Reyner D, Rossing P, Sjöström CD, Wheeler DC, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol, 2019; 7(6): 429-41. https://doi.org/10.1016/S2213-8587(19)30086-5
12. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med, 2020; 383(15): 1436-46. https://doi.org/10.1056/NEJMoa2024816
13. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med, 2019; 380(24): 2295-306. https://doi.org/10.1056/NEJMoa1811744
14. Suijk DLS, van Baar MJB, van Bommel EJM, Iqbal Z, Krebber MM, Vallon V, et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Clin J Am Soc Nephrol, 2022; 17(5): 663-71. https://doi.org/10.2215/CJN.11480821
15. Mahling M, Schork A, Nadalin S, Fritsche A, Heyne N, Guthoff M. Sodium-glucose cotransporter 2 (SGLT2) inhibition in kidney transplant recipients with diabetes mellitus. Kidney Blood Press Res, 2019; 44(5): 984-92. https://doi.org/10.1159/000501854
16. Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia, 2019; 62(7): 1154-66. https://doi.org/10.1007/s00125-019-4859-4
17. McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Cardiol, 2019; 124: S45-52. https://doi.org/10.1016/j.amjmed.2019.08.006
18. Francis RS. SGLT2i after kidney transplantation: ready for prime time? Transplantation, 2022; 106(9): 1732-3. https://doi.org/10.1097/TP.0000000000004227
19. Lim JH, Kwon S, Jeon Y, Kim YH, Kwon H, Kim YS, et al. The efficacy and safety of SGLT2 inhibitors in diabetic kidney transplant recipients. Transplantation, 2022; 106(9): e404-12. https://doi.org/10.1097/TP.0000000000004228
20. Schwaiger E, Burghart L, Signorini L, Ristl R, Kopecky C, Tura A, et al. Empagliflozin in posttransplantation diabetes mellitus: a prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant, 2019; 19(3): 907-19. https://doi.org/10.1111/ajt.15223
21. Halden TAS, Kvitne KE, Midtvedt K, Rajakumar L, Robertsen I, Brox J, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care, 2019; 42(6): 1067-74. https://doi.org/10.2337/dc19-0093
22. Shah M, Virani Z, Rajput P, Shah B. Efficacy and safety of canagliflozin in kidney transplant patients. Indian J Nephrol, 2019; 29(4): 278-81. https://doi.org/10.4103/ijn.IJN_2_18
23. Sánchez Fructuoso AI, Bedia Raba A, Banegas Deras E, Vigara Sánchez LA, Valero San Cecilio R, Franco Esteve A, et al. Sodium-glucose cotransporter-2 inhibitor therapy in kidney transplant patients with type 2 or post-transplant diabetes: an observational multicentre study. Clin Kidney J, 2023; 16(6): 1022-34. https://doi.org/10.1093/ckj/sfad007
24. Agarwal R. Pathogenesis of diabetic nephropathy. ADA Clinical Compendia, 2021; 2021(1) :2-7. https://doi.org/10.2337/db20211-2
25. Attallah N, Yassine L. Use of empagliflozin in recipients of kidney transplant: a report of 8 cases. Transplant Proc, 2019; 51(10): 3275-80. https://doi.org/10.1016/j.transproceed.2019.05.023
26. Ujjawal A, Schreiber B, Verma A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: what is the evidence? Ther Adv Endocrinol Metab, 2022; 13. https://doi.org/10.1177/20420188221090001
27. AlKindi F, Al-Omary HL, Hussain Q, Al Hakim M, Chaaban A, Boobes Y. Outcomes of SGLT2 inhibitors use in diabetic renal transplant patients. Transplant Proc, 2020; 52(1): 175-8. https://doi.org/10.1016/j.transproceed.2019.11.007
28. Oliveras L, Montero N, Cruzado JM. Searching in the maze: sodium-glucose cotransporter-2 inhibitors in kidney transplant recipients to improve survival. Clin Kidney J, 2023; 16(6): 909-13. https://doi.org/10.1093/ckj/sfad045
29. Gibson C, Mount R, Valentine H, Sullivan D. Weight change in kidney transplant recipients: an academic medical centerbased study. Curr Dev Nutr, 2020; 4:nzaa063_035. https://doi.org/10.1093/cdn/nzaa063_035
30. Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol, 2021; 83(1): 503-28. https://doi.org/10.1146/annurev-physiol-031620-095920
31. Chewcharat A, Prasitlumkum N, Thongprayoon C, Bathini T, Medaura J, Vallabhajosyula S, et al. Efficacy and safety of SGLT-2 inhibitors for treatment of diabetes mellitus among kidney transplant patients: a systematic review and meta-analysis. Med Sci, 2020; 8(4): 47. https://doi.org/10.3390/medsci8040047
32. Lemke A, Brokmeier HM, Leung SB, Mara KC, Mour GK, Wadei HM, et al. Sodium-glucose cotransporter 2 inhibitors for treatment of diabetes mellitus after kidney transplantation. Clin Transplant, 2022; 36(8): e14718. https://doi.org/10.1111/ctr.14718
33. Polychronopoulou E, Bourdon F, Teta D. SGLT2 inhibitors in diabetic and non-diabetic kidney transplant recipients: current knowledge and expectations. Front Nephrol, 2024; 4. https://doi.org/10.3389/fneph.2024.1332397
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2025 Matheus Rizzato Rossi, Leticia Harumi Richter Kawai , Marilda Mazzali, Marcos Vinícius de Sousa

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.